Magic Molecules
Freiburg, Oct 12, 2017
Jennifer Andexer is a junior professor at the Institute of Pharmaceutical and Medicinal Chemistry at the University of Freiburg. Although the thirty-seven year old got her degree in biology, she dared enter the field of chemistry after her PhD – first in Cambridge, England, then later in Freiburg. The fact that biochemist can maneuver between two disciplines got her a Starting Grant from the European Research Council (ERC) and with it 1.5 million euros in funding for her efforts. The University of Freiburg is celebrating the tenth anniversary of the ERC grant and its 50 recipients with a look at select projects: A series introduces portraits form ten thinkers.

Jennifer Andexer has a degree in biology and after her dissertation dared to enter the field of chemistry – the fact that the researcher can work at the intersection between two fields got her an ERC Starting Grant. Photo: Thomas Kunz
Jennifer Andexer likes to develop methods with which substances can be changed with the help of special enzymes that make them more effective or taste more intensely. Medication, for instance, or flavors in the food industry. Enzymes, what was that again? Andexer explains: “An enzyme is always a protein. A protein is a succession of amino acids that are ordered like a string of pearls.” Enzymes are at work in lactose-free milk, for example. They ensure that lactose is broken down into digestible bits. In nature, enzymes don’t exist in a simple chain, however. Rather the string with the amino acids is folded three-dimensionally and gives the enzyme a particular form. It ensures that enzymes act as biocatalysts; that is, they accelerate biochemical reactions. Enzymes are somehow magic. A bit like a magical machine in which you put something into it and out comes your magic wish. Andexer relativizes: Of course you have to know exactly how the enzyme functions with which you are working. “But it’s cool nonetheless.”
Keeping the cycle going
There are also enzymes that require so-called cofactors in order to be able to function as a catalyst. If enzymes and cofactors come together, the enzyme can transfer, for instance, a chemical group from the cofactor onto a target molecule. What is left over is a cofactor without this group. A cofactor on which Andexer and her team are working is called S-adenosylmethionine. This molecule consists of a lot of carbon, nitrogen, hydrogen and a bit of sulfur without oxygen. But a methyl group belongs to it that is made up of one carbon atom and three hydrogen atoms – that is the group that is transferred.
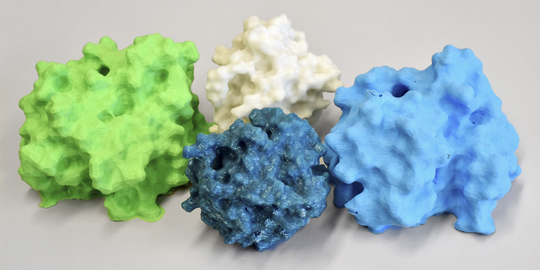
Colorful, round and messengers of hope for biotechnology: Enzymes that transfer methyl groups can seriously intensify the effectiveness of medication or flavors like vanilla. Photo: Thomas Kunz
So far, so good. Except: What is left over after the reaction from the cofactor can hinder the enzyme. “These byproducts from the cofactor start to gather and stop up the enzyme’s so-called active center so that its effectiveness is hindered,” explains Andexer. Another issue: The reaction cannot be kept up without the cofactors’ reinforcements. In order to ensure everything is working properly, Andexer and hear team have combined multiple reaction cycles with one another. The researchers had to do a lot of experimenting to get to that point. A further molecule now ensures reinforcements and gives back the missing group to the residual cofactor so that it can react again with the first enzyme. In order to keep costs low, manufacturing the cofactor should be as inexpensive as possible. “We have to think of that as well,” says Andexer. “After all, we need a lot of it.” The team uses enzymes from natural organisms. Why not simply synthesize chemically? “The structure of such cofactors is extremely complex,” explains the biochemist. And why not carry out the actual reaction chemically? It would be possible and, depending on the desired reaction even sensible, but in some cases only under the use of toxic substances.
The more vanilla-like vanilla
In addition, enzymes can often control the reaction better than chemical catalysts. It depends on their three-dimensional structure. The reactive partners can only organize themselves and interact in a single fashion in the active center. “The enzyme does it itself. During chemical synthesis the group could also dock on somewhere else completely.” With the result that you don’t get what you wished for.
Okay. So it’s not a wish machine after all, but rather a product dependent on many variables. But back to the enzymes that Andexer explores – the so-called methyl transferases (because they transfer methyl groups). “The effectiveness of these methyl groups in particular can be huge,” she says. When placed correctly, they can raise the effectiveness of pharmaceuticals a thousandfold, for instance. And a flavor like vanilla suddenly smells a lot more vanilla-like. Magic Methyl Effect is what they call it in technical terms. Now, that’s magic.
Stephanie Streif

