Muscle recovery
Freiburg, Jan 26, 2018
Skeletal muscles enable breathing and movement, but also play a significant role in heat production and metabolism. It is therefore essential that damaged muscle is quickly and efficiently repaired to allow proper function of the whole organism. A research team led by Prof. Dr. Roland Schüle and Milica Tosic has now proven that the epigenetic enzyme lysine-specific demethylase (Lsd1) plays a key role in the regeneration of skeletal muscle and specification of muscle stem cells. The researchers recently published their findings in the journal Nature Communications.
Muscle stem cells, termed satellite cells are located beneath the so-called basement membrane, which encases the muscle cells, and have the ability to develop into muscle cells as well as into brown fat cells. Up to now, however, the molecular mechanisms that regulate these processes have not been entirely known.
The Freiburg team injected genetically modified mice with snake venom, thereby causing muscle injury. They showed that an increased Lsd1 level accelerates muscle regeneration because the enzyme triggers the expression of genes that promote muscle formation. In contrast, a lack of Lsd1 or inhibition of its activity resulted in delayed muscle regeneration and caused satellite cells to turn into fat cells instead of muscle. These cells not only produce lipid coating protein perilipin 1, but also uncoupling protein 1, found exclusively in brown fat cells. In order to prove that these cells originated from satellite cells, the scientists tagged the stem cells with a green fluorescent protein and were thus able to track the formation of brown fat cells during the regeneration process.
At the molecular level, the researchers showed that Lsd1 prevents the development of satellite cells to brown fat cells by inhibiting the protein GLIS family zinc finger 1 (Glis1). In contrast, elevated levels of Glis1 in mice and in in vitro experiments were sufficient to promote differentiation into brown adipocytes, thus mimicking the effect of Lsd1 deletion. In summary, suppression of Glis1 and concomitant up-regulation of muscle specific genes ensure muscle recovery.
Roland Schüle and Milica Tosic conduct their research in the Department of Urology and in the Clinical Research Center at the Medical Center – University of Freiburg. In addition, Schüle is a member of the Cluster of Excellence BIOSS Centre for Biological Signalling Studies.
Original publication:
Milica Tosic, Anita Allen, Dominica Willmann, Johnny Kim, Christopher Lepper, Delphine Duteil, Roland Schüle (2018). Lsd1 regulates skeletal muscle regeneration and directs the fate of satellite cells. NatComm. DOI: 10.1038/s41467-017-02740-5
Report about Roland Schüle’s research in uniˈwissen (only in German)
www.pr2.uni-freiburg.de/publikationen/uniwissen/uniwissen-2014-1/#/8
Contact:
Prof. Dr. Roland Schüle
Department of Urology and Clinical Research Center
Medical Center – University of Freiburg
BIOSS Center for Biological Signalling Studies
Tel.: 0761/203-63100
E-Mail: roland.schuele@uniklinik-freiburg.de
Milica Tosic
Department of Urology and Clinical Research Center
Medical Center – University of Freiburg
Tel.: 0761/203-63360
E-Mail: milica.tosic@uniklinik-freiburg.de
Graphic for download
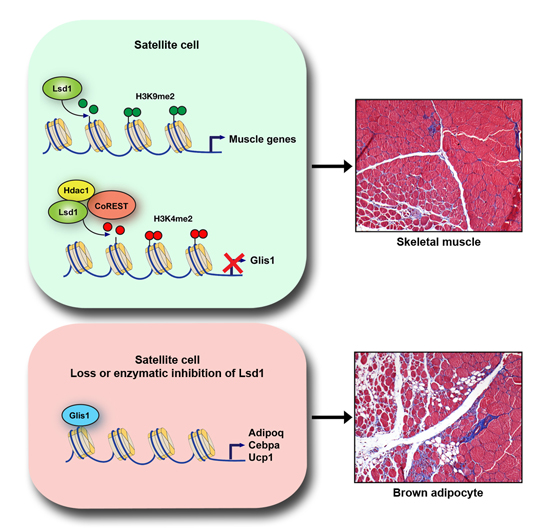
Lsd1 activates gene expression in certain stem cells, thereby accelerating the regeneration of the muscle (top). Enzyme removal causes the cells to turn into fat cells rather than muscle cells (bottom). Source: Milica Tosic

