Saving lives and money
Freiburg, Jan 25, 2019
33,000 people die from antibiotic-resistant germs annually in the EU. "Three out of every four people affected are infected in hospitals," said Dr. Mark Keller from SpinDiag GmbH. The spin-off from the Institute of Microsystems Engineering (IMTEK) at the University of Freiburg and the Hahn-Schickard hopes to prevent infection. However, some tests take several days during which time the resistant germs can spread more and more within the hospital. In contrast, SpinDiag's test system delivers such results within 30 minutes.
 33,000 people die every year in the European Union from antibiotic-resistant germs - three out of four afflicted people get infected in clinics. Photo: spotmatikphoto/stock.adobe.com
33,000 people die every year in the European Union from antibiotic-resistant germs - three out of four afflicted people get infected in clinics. Photo: spotmatikphoto/stock.adobe.com
The simple, inexpensive system could save lives and money, says Keller: "We expect to obtain EU approval in 2020. A multi-center study with SpinDiag technology will be running next year. After that, we still have to advertise and sell it." That should be easy: At the beginning of the development phase, there was a market survey among health care facilities about the possible fields of application of the technology: most notably, clinics and nursing homes indicated that there was an urge to be able to identify and isolate carriers of resistant bacteria quickly. This would make it easy to reduce the number of infections. With around 2,400 cases per year, Germany is still quite well placed compared to the rest of Europe. But the misery has a rising tendency everywhere: Infections and deaths due to antibiotic resistance have doubled since 2007.
Potential carriers are 40 percent of all new admissions to hospitals. These include people requiring dialysis, people with frequent contact with pigs, cattle and poultry, and people who have received antibiotics in the last six months. On the outside, it is almost impossible to tell who actually brings resistant germs with them. Only detection tests can provide certainty. However, the usual tests sometimes take three days. To be absolutely certain, clinics would have to keep their other patients away from the suspects for that long: The real carriers among them could pass on otherwise resistant bacteria during this time - for example through shared sanitary facilities.
 Insert disc and press "Start": Mark Keller presents the player that reads the result. The system currently identifies 25 important resistant pathogens. Photo: Thomas Kunz
Insert disc and press "Start": Mark Keller presents the player that reads the result. The system currently identifies 25 important resistant pathogens. Photo: Thomas Kunz
A dilemma between protection and cost
Keeping away means quarantine, which results in enormous expenses, including single rooms, restricted access for doctors, nursing staff and relatives, protective gowns and disposable gloves, daily disinfection of laundry and personal items. Such measures cost more than 300 euros per day for intensive care patients and 100 to 200 euros for others. “But only five percent of those at risk actually have resistant pathogens,” Keller emphasizes, “They are the only ones who have to be quarantined.”
The clinics have a serious dilemma: they have to protect their patients, but they also want to keep costs low. Many have to make compromises that increase the risk of infection. Infections caused by resistant pathogens are most threatening for people with severely weakened or suppressed immune systems, such as after transplants. “Our system increases patient safety and profitability immensely,” says Keller. He is holding a crescent-shaped disc about one centimeter thick and slightly larger than a CD in half: “This is the disc - it is smart and the core of our technology.”
Centrifugal force make the samples flow
On the straight side of the disc there is an integrated standard swab for patient samples. It is like an ear stick, but with only one cotton swab, that allows nurses to take a swab on suspected infected individuals. Then the users push the swab inside the disc, close it and insert it into a processing device, which is called the player. “Its application is so easy that nursing staff can do everything on their own,” says Keller. Press “start” to rotate the disc in the player. Centrifugal forces act on the disc, in which weak microchambers and microchannels are visible from the outside. A preparation liquid flows from certain chambers to the sample. The mixture is distributed over the channels in twelve chambers. Currently, the system identifies 25 important resistant pathogens if the patient sample contains one or more of them.
The player reads the result. Positive reactions produce fluorescent light signals. The position of the chamber and the color in which it illuminates reveal whether and which resistant germs are present. “All results are automatically sent to the hospital information system,” says Keller. Doctors can view the findings in the electronic patient file just half an hour after entering the disc. “Our test series corresponds to the wish list of the national reference laboratories in the EU,” he states.
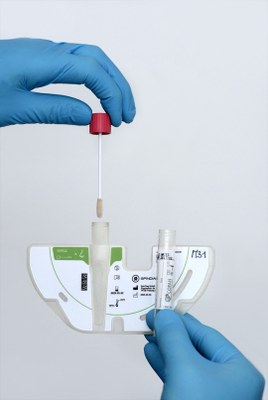
The disk is the core of the SpinDiag technology: With the swab, nurses can take samples from patients who may be infected. Photo: Thomas Kunz
The plans emerged at the regulars’ table
SpinDiag emerged in 2016 from the IMTEK Professorship for Application Development through the Hahn-Schickard Institute Director Prof. Dr. Roland Zengerle. In addition to product manager Keller, the founding team also includes production manager Dominique Kosse, managing director Dr. Daniel Mark, technology manager Dr. Oliver Strohmeier, biochemistry expert Dr. Gregor Czilwik and fluidics expert Dr. Frank Schwemmer. The sextet had already started meeting regularly during their time at university, says Keller: “We wanted to build something with our technology.” Centrifugal microfluidics is the name of the principle that uses rotation to move fluids through tiny channels just like in a disc. SpinDiag is currently adapting the system so that it can also be used for other applications such as the diagnosis of sexually transmitted diseases, resistant tuberculosis and other respiratory diseases. “This would not require a new player, but only a different disc,” explains Keller.
“Technologically, we are literally running smoothly,” he says happily. “The positive response from the clinics also gives us a lot of hope.” Following EU approval, SpinDiag is aiming for approval for the US market. The technology is comprehensively protected by many patents.
Jürgen Schickinger

