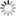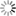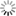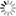Sugar-based inhibitors disarm the pathogen Pseudomonas aeruginosa
Freiburg, Jan 17, 2023
LecA (green) binds to the surface of host cells (left). When one of the newly developed inhibitors (right) is added, the binding of LecA is prevented (scale bar = 10 μm). Image: Francesca Rosato, Dorina Reith/University of Freiburg
The hospital pathogen Pseudomonas aeruginosa requires the sugar-binding proteins LecA and LecB to form biofilms as well as to attach to and penetrate host cells. These so-called lectins are therefore suitable targets for active substances to combat Pseudomonas infections. Researchers from Saarbrücken and Freiburg have now produced potent inhibitors for LecA and LecB that are more stable and soluble than previous drug candidates. These optimized molecules have been tested in virulence assays and show promising properties for the development of new drugs.
Pseudomonas aeruginosa is a Gram-negative bacterium that belongs to the group of highly antibiotic-resistant and clinically relevant ESKAPE pathogens. According to the World Health Organization (WHO), it is one of the critical priority pathogens because it is often resistant to antibiotics and lacks treatment options. P. aeruginosa produces two lectins, LecA and LecB, which it uses to bind to sugar molecules. It requires them for adhesion to host cells and for biofilm formation - two properties that are critical to the bacterium's pathogenicity. This makes these lectins promising targets for synthetic agents that counteract the bacterium's disease-causing properties and bypass its antimicrobial resistance.
Prof Dr Alexander Titz's research group at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) and Saarland University is investigating molecules to inhibit bacterial lectins, particularly from P. aeruginosa. HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University. The group, which is also part of the German Center for Infection Research (DZIF), had already developed highly potent LecA inhibitors, but they had low water solubility and limited stability, which prevented their further biological analysis. The current results come from a joint research project with Prof Dr Winfried Römer's group at the University of Freiburg and the Cluster of Excellence CIBSS - Centre for Integrative Biological Signalling Studies. Römer and his team are investigating how the lectins LecA and LecB of P. aeruginosa influence physiological processes such as immune response and wound healing, and how this can be prevented by inhibitors.
The researchers have now succeeded in significantly improving the chemical properties of previous lectin inhibitors. The targeted modification of linker regions within the molecules increased their solubility by a factor of 1000 and enhanced their chemical and metabolic stability. The results of the collaborative project have been published as a “hot paper” in the renowned journal Angewandte Chemie.
The greatly increased solubility of the lectin inhibitors made it possible to evaluate their biological effect in cell-based assays. "We investigated the influence of the inhibitors on the biological function of LecA and the invasiveness of P. aeruginosa," Römer explains. "We were able to show that even relatively low concentrations of the LecA inhibitor are sufficient to prevent P. aeruginosa from invading host cells." Thus, the inhibitors effectively block the binding of LecA to human cells. "In addition to these positive results in the cell assays, we also saw that the new inhibitor can be detected in urine for a long time, potentially opening up further treatment options for urinary tract infections," said Titz, head of the Chemical Biology of Carbohydrates research group at HIPS.
These results show that the developed drug candidates have the potential to circumvent antibiotic resistance of dangerous pathogens. In particular, inhibition of LecA-mediated virulence offers a promising starting point for new agents to treat often highly problematic infections with P. aeruginosa. The current results are a first step and open the door for the future development of new treatment options.
Excellence Cluster CIBSS:
The objective of the Excellence Cluster CIBSS – Centre for Integrative Biological Signalling Studies at the University of Freiburg is to gain comprehensive understanding of biological signalling procedures regardless of scale, from interactions between individual molecules and cells to processes within organs and entire organisms. The researchers deploy the knowledge they obtain to develop strategies for targeted signal control. These technologies pave the way for new research findings and enable innovations in medicine and plant sciences. www.cibss.uni-freiburg.de2
Overview of facts:
- Original Publication: Zahorska, E., Rosato, F., Stober, K., Kuhaudomlarp, S., Meiers, J., Hauck, D., Reith, D., Gillon, E., Rox, K., Imberty, A., Römer, W.,* Titz, A.* (2022): Neutralizing the Impact of the Virulence Factor LecA from Pseudomonas aeruginosa on Human Cells with New Glycomimetic Inhibitors. In: Angew. Chem. Int. Ed. Engl., DOI: 10.1002/anie.2022155353.
- Winfried Römer is Professor of Synthetic Biology of Signaling Processes at the Faculty of Biology of the University of Freiburg and member of the Cluster of Excellence CIBSS. His research focuses on host-pathogen interactions.
- Alexander Titz is Professor of Organic and Pharmaceutical Chemistry at Saarland University in Saarbrücken and research group leader at HIPS. His research focuses on medicinal chemistry-based development of novel anti-infectives and diagnostics, as well as the biochemical and microbiological characterization of bacterial lectins involved in infection.
- The study was supported by: J. Konstantinovic (HIPS, Saarbrücken), J. Wolf and K. V. Sander, German Centre for Infection Research (DZIF, TTU 09.710 and 09.719), European Research Council (ERC Starting Grant, Sweetbullets) and the DZIF (TTU 09. 718), French National Research Agency [ANR-17-CE11-0048], Labex Arcane/CBH-EUR-GS (ANR-17-EURE-0003), EU Research and Innovation Programme Horizon 2020 under the Marie Skłodowska-Curie funding synBIOcarb (no. 814029), COST (European Coorperation in Science and Technology), German Research Foundation (DFG) in the framework of the Excellence Strategy (EXC-294 and EXC-2189) and DFG funding Large-scale Research Facilities (project number: 438033605) by the Ministry of Science, Research and the Arts of the State of Baden-Württemberg (Ref: 33-7532.20) and by the Freiburg Institute for Advanced Studies.
Contact:
Annette Kollefrath-Persch
Office of University and Science Communications
University of Freiburg
Tel.: 0761/203-8909
e-mail: annette.persch@zv.uni-freiburg.de5