New strategy for clinically relevant protein sequencing
Freiburg, May 09, 2023
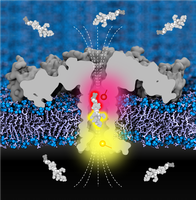
See text for image caption. Source: Tobias Ensslen, Physiologisches Institut der Universität Freiburg, mit Material von Dr. Kumar Sarthak, Center for Biophysics and Quantitative Biology, University of Illinois at Urbana-Champaign/USA
Proteins have characteristic amino acid sequences, the analysis of which is fundamental for research and medicine. These can be decoded; however, so-called protein sequencing is expensive and time-consuming. A large-scale research project led by Prof. Dr. Jan Behrends from the Institute of Physiology at the University of Freiburg now aims to establish a new technology for protein sequencing using nanopores, which will be rapid and cost-effective. The project “Nanopore-based Electro-Optical Protein Diagnostics” (nEOdiag) is funded by the Carl-Zeiss-Stiftung with five million euros; it will start in the fall of 2023 and run for six years.
Electrical signals and fluorescence
“Protein sequencing promises to significantly advance research and medicine,” says Behrends. Proteins are folded chain molecules consisting of 21 different amino acids. Their characteristic sequence to a large extent determines their form and function. Therefore, the analysis of these sequences is of fundamental importance – but it is also much more complex than the sequencing of DNA, which consists of only four building blocks. “Our protein diagnostics, which rely on the simultaneous measurement of electrical signals and fluorescence using nanopores, could lead to significant progress here,” says Behrends.
Nanopores are holes with molecular dimensions in an electrically insulating material. When amino acid chains enter such a pore through which ions flow, a current signal is measured electrically. In addition, the inner pore wall is provided with fluorescent molecules whose light signal is characteristically altered by the amino acids directly interacting with them in a very confined space. The correlation of the two signals will now enable rapid, inexpensive and reliable protein sequencing with nanopores for the first time. However, practical implementation of this idea requires microelectronic innovations for highly parallel, multimodal data acquisition, AI-based algorithms for sensor fusion-based recognition of protein sequences, and microsystems engineering innovations in miniaturization, parallelization, and integration.
Highly interdisciplinary approach
To this end, the project takes a highly interdisciplinary approach: In addition to Behrends as spokesperson, the project involves biophysicist Dr. Maximilian Ulbrich from the Department of Internal Medicine IV at the Medical Center – University of Freiburg and the Medical Faculty of the University of Freiburg, computer scientist Prof. Dr. Oliver Amft, as well as microsystems engineers Prof. Dr. Matthias Kuhl and Prof. Dr. Roland Zengerle from the Faculty of Engineering at the University of Freiburg as well as Prof. Dr. Felix von Stetten from the Hahn-Schickard Institute for Microanalysis Systems.
Among other things, the project also supports a junior professorship that is intended to establish single-molecule analysis at the University of Freiburg in the long term. Behrends has already been successful with previous projects on nanopore technology, for example with the thematically related future cluster nanoDiag BW. However, the research and development in the cluster focuses on the detection of epigenetic modifications, in particular of histone proteins, pursues the electrical approach alone for the optimization of sensor technology and has a clear focus on sample preparation. The disproportionately more complex multimodal electrical-optical nanopore sensing is unique to the new nEOdiag project and crucial for the protein sequencing strategy.
About the Carl-Zeiss-Stiftung
The Carl-Zeiss-Stiftung’s mission is to create an open environment for scientific breakthroughs. As a partner of excellence in science, it supports basic research as well as applied sciences in the STEM subject areas (science, technology, engineering and mathematics). Founded in 1889 by the physicist and mathematician Ernst Abbe, the Carl-Zeiss-Stiftung is one of the oldest and biggest private science funding institutions in Germany. It is the sole owner of Carl Zeiss AG and SCHOTT AG. Its projects are financed from the dividend distributions of the two foundation companies.
Carl-Zeiss-Stiftung press release
Overview of facts:
- The project “Nanopore-based Electro-Optical Protein Diagnostics” (nEOdiag) has a volume of more than six million euros, it is funded by the Carl-Zeiss-Stiftung with five million euros. The project will run from October 2023 to September 2029.
- Spokesperson Jan C. Behrends is Professor of Physiology at the Medical Faculty of the University of Freiburg. His research interests include single molecule detection and characterization of synthetic and natural polymers with nanopores as well as method development for electrophysiology.
Press image for download
Contact:
Office of University and Science Communications
University of Freiburg
Tel.: 0761/203-4302
E-Mail: kommunikation@zv.uni-freiburg.de