Freiburg physiologists find keys to kidney function and sensory perception process
Freiburg, Jan 23, 2023
Physiologists investigate functions and processes in human, animal or plant organisms. At the Faculty of Medicine of the University of Freiburg researchers are delving into, among other things, the filtering function of the human kidney. They are also analyzing the molecular structure of the activation mechanism of transient receptor potential (TRP) channels in the nervous system, which process sensory perception.
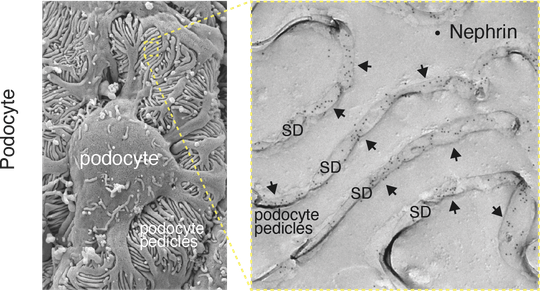
Left: Scanning EM picture of the podocytes engulfing blood vessels with their foot processes (pedicles), Right: EM image of a freeze-fracture showing the pedicles and the ‘slit diaphragm’ (SD) between them. The Nephrin proteins of the SD are stained with antibodies carrying gold-particles (dots in black).
The slit-diaphragm in the kidney: a sensory network – not a filter
The filtration of blood is of fundamental significance to the life of all mammals. It is how metabolic end products and toxins are effectively removed from the blood and excess water and electrolytes rapidly excreted. Filtration takes place in the kidneys. It must continually adjust to the mammal’s lifestyle and nutrition. As the last of three components of the kidney filter, the slit diaphragm is a key to this vital process. Up to now, for technical reasons it was not possible to explain which proteins make up the slit diaphragm and how they regulate filtration. A team working with Freiburg researcher Prof. Dr. Bernd Fakler and Dr. Maciej Kocylowski of the Institute of Physiology and Dr. Florian Grahammer of the University Hospital Hamburg-Eppendorf (UKE) have identified the protein components of the slit diaphragm of a mouse. They have also more precisely defined the structure’s function.
With the help of high resolution proteomic analysis and quantitative mass spectrometry, the researchers were able to show the slit diaphragm is made up of the components Neprhin, Neph1, and Podocin as well as a previously unknown high-molecular weight protein network. “This network has a double function,” says Kocylowski. He explains, “It serves as an anchor for the slit spanning proteins Nephrin and Neph1. At the same time, it connects the slit-forming part of the membrane with the cytoskeleton of the cell (podcyte) to which it belongs and the signalling pathways that run within it.”
The slit diaphragm functions as a sensory system during blood filtration
The researchers’ results of proteomic and functional analyses additionally proved the slit diaphragm is not a passive filter. Instead, it is a complex sensory system for dynamic regulation of blood filtration in the kidney. The significance of this network became clear when Grahammer and his research team removed individual parts of this newly identified network and discovered that filtration was impeded or completely shut down.
“This system probably lends kidney filtration a context-dependent dynamic required for the adaptation of the filter to the demands of continually changing conditions,” explains Fakler. The researchers plan now to build on this work by investigating the newly identified slit diaphragm components in order to gain understanding of precisely how they regulate kidney function. They will also examine their role in worsening of kidney function (renal failure) and the life-threatening losses of protein caused by it.
Comprehending the processing of sensory impressions
During the processing of daily sensory impressions such as taste, sight, pain or warmth, what are known as transient receptor potential channels (TRP channels) take part. They are located, among other places, in the cells of our nervous system. In 2021, David Julius and Ardem Patapoutian were awarded the Nobel Prize for Medicine for discovering and researching them. Freiburg researchers working with Bernd Fakler and Dr. Astrid Kollewe and her colleagues at Saarland University have now succeeded in deciphering the molecular structure of TRPC channels and their activation mechanism in the brains of mice. TRPC channels are known as the “classic subfamily” of the TRP channels. They play an important role in passing on information between nerve cells. Defective TRPC channels can cause movement disorders or kidney diseases.
TRPC channels are integrated in larger protein complexes
By using techniques of high resolution proteomic analysis, the research team could determine the composition of the TRPC channels in the brains of mice. “Our experiments have shown that TRPC channels in the brain are not present as isolated channel pores. Instead, they must be integrated with further proteins in larger complexes,” Kollewe explains. “Additionally, we saw during the assembly of the TRPC proteins to channel pores, that not all the experimentally possible combinations were used and different TRPC subclasses have very different affinities to forming mixed or more uniform channel pores,” she adds. Beyond that, the researchers identified a further 15 proteins which interact with sub-classes of the TRPC channels in the brains of mice. “Most of these proteins hadn’t been brought into connection with TRPC channels before at all,” says Fakler.
The researchers were also able to show the following regarding the activation mechanism of the TRPC channels: Ca2+ (Calcium ions (Ca2+)) must be released in direct vicinity of the TRPC channel in order to stimulate them. “Even if the activation can theoretically take place without direct interaction between the Ca2+-releasing receptor and the TRPC channel, the naturally selected complex formation between both likely serves to make signalling transmission more reliable,” elaborates Fakler. “Activation is also spatially limited and signalling transmission accelerated.”
Factual overview:
- Original publications:
- Kocylowski MK, Aypek H, Bildl W, Helmstädter M, Trachte P, Dumoulin B, Wittösch S, Kühne L, Aukschun U, Teetzen C, Kretz O, Gaal B, Kulik A, Antignac C, Mollet G, Köttgen A, Göcmen B, Schwenk J, Schulte U, Huber TB, Fakler B, Grahammer F. A (2022): A Slit-diaphragm-associated protein network for dynamic control of renal filtration. In: Nature Communications. DOI: https://doi.org/10.1038/s41467-022-33748-1
- Kollewe A, Schwarz Y, Oleinikov K, Raza A, Haupt A, Wartenberg P, Wyatt A, Boehm U, Ectors F, Bildl W, Zolles G, Schulte U, Bruns D, Flockerzi V, Fakler B (2022). Subunit composition, molecular environment, and activation of native TRPC channels encoded by their interactomes. In: Neuron. DOI: https://doi.org/10.1016/j.neuron.2022.09.029
- Since 2001, Falker has been the head of Department II of the Institute of Physiology of the Faculty of Medicine of the University of Freiburg.
- Fakler’s research is focused in particular on molecular organization and function of the rapid signal transduction at the cell membrane, proteomic analysis of membrane proteins, and the structural and functional analysis of ion channels and G protein-coupled receptors.
- He is a member of the Cluster of Excellence, the CIBSS – Centre for Integrative Biological Signalling Studies and of the BIOSS – Centre for Biological Signalling Studies.
- Fakler was the spokesperson for Collaborative Research Center 746, which examined functional specificity by coupling and modification of proteins until its work was successfully concluded in 2018.

