Until the Tipping Point is Reached
Freiburg, Oct 12, 2020
Most people know that too much fast food and too little exercise are not healthy in the long term. What is less well known is that this lifestyle, which is common in many countries, also influences our immune system. Allergies, asthma, cancer – diseases associated with the immune system – are becoming more prevalent. The Freiburg medical researcher Dr. Nina Chevalier wants to find out how diet and other environmental factors trigger immunological mechanisms – causing the immune system to attack the body’s own cells. The doctor and scientist is concentrating particularly on two rheumatological autoimmune diseases in her research: rheumatoid arthritis and systemic lupus erythematosus.
 Painful arthritis: A Freiburg medical researcher wants to find out how our diet and environmental factors trigger immunological mechanisms. Photo: Anut21ng Photo/stock.adobe.com
Painful arthritis: A Freiburg medical researcher wants to find out how our diet and environmental factors trigger immunological mechanisms. Photo: Anut21ng Photo/stock.adobe.com
When pathogens like bacteria or viruses get into the body, the immune system initially mobilizes phagocytes to limit the damage. Simultaneously, specific immune cells in the lymphatic organs like the lymph nodes begin to reproduce. These cells identify the marauding invaders and neutralize them. The problem is that the mobilization does not always go off as planned. Autoreactive cells that attack the body itself can also be activated – also because foreign pathogens can be very similar to the body’s own structures. Nina Chevalier refers to this error in the system as a “tolerance break.” Tolerating the body’s own structures and distinguishing them from foreign structures are characteristic features of the specific immune response.
Autoreactive cells do not cause disease automatically, because a healthy body can also suppress them. “Occasionally, however, there is an autoimmune response with pathological significance,” Chevalier explains. This response can be triggered by the interaction between genes and environmental influences. Factors like an unhealthy diet, stress, pollutants, or an infectious pathogen are often what causes the system to reach the tipping point. The consequence is inflammations. The body does what it always does when it is attacked by germs or other intruders: it gets fired up to defend itself. But inflammations that no longer subside – the result of a dysregulated immune system – damage the tissue. Autoimmune diseases are not curable, but they can be treated through large-scale suppression of the overregulated immune system. If scientists were to succeed in deciphering the underlying mechanisms at the molecular level, it would be possible to intervene in a much more targeted way, for example by blocking only individual molecules. A few drugs already do this.
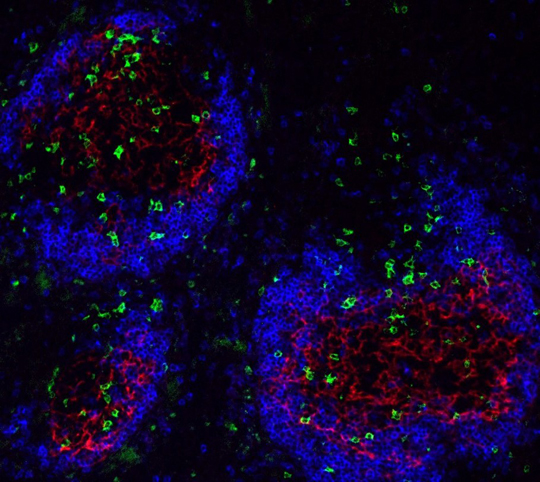 Specific T lymphocytes (marked green) migrate into the germinal centers (marked blue-red), where they support the production of autoantibodies. Illustration: Nina Chevalier, DOI 10.1002/art.39481
Specific T lymphocytes (marked green) migrate into the germinal centers (marked blue-red), where they support the production of autoantibodies. Illustration: Nina Chevalier, DOI 10.1002/art.39481
Components of a Chain Reaction
Chevalier is also studying molecules in the context of her research: “I’m interested in what the environmental factor does with the immune cells X, Y, and Z and why that then triggers a disease.” Chevalier works with mouse models. In a mouse model on rheumatoid arthritis, for instance, it is possible to trigger the disease by bringing together two components, she explains: a specific T cell (defense cell) and one of the body’s own antigens (autoantigen) that is recognized by this T cell. When the two meet, the pathogenic chain reaction is set in motion: The autoantigen is recognized, which results in the T cell being activated and interacting with other immune cells, such as B cells. “This in turn leads to the B cells expanding, differentiating, and producing autoantibodies,” explains Chevalier. The consequence is arthritis.
Boosters in the Molecular Interplay
Next, the scientists manipulated the mice, which carried both components through cell transfer, with diseases caused by infectious pathogens – such as intestinal inflammation or influenza with pneumonia. And indeed, says Chevalier, this boosted the arthritis. The mice’s paws turned red and swelled up. Chevalier succeeded in identifying the boosters: soluble factors such as the messenger interleukin 1 beta, which triggers the formation of pro-inflammatory T helper 17 cells. “They were highly regulated in our influenza and colitis mice.” Chevalier also managed to prove that these factors act as boosters in the molecular interplay – by knocking out the receptors responsible for recognizing these factors and thus blocking the signaling processes inside the cell: there was no information and thus no response and no inflammation.
 Pulses for a healthy diet: Dietary fibers are broken down in the intestines into short-chain fatty acids, which also have anti-inflammatory effects on immune cells. Photo: zerbor/stockk.adobe.com
Pulses for a healthy diet: Dietary fibers are broken down in the intestines into short-chain fatty acids, which also have anti-inflammatory effects on immune cells. Photo: zerbor/stockk.adobe.com
The Benefit of Fiber
This is just one aspect of Chevalier’s work. In mice with lupus erythematosus, she is also studying the effect of a fiber-rich diet. “The dietary fiber we ingest is broken down into short-chain fatty acids in the intestines,” says the Freiburg scientist – and they have anti-inflammatory effects, for instance on the immune cells. They also improve the production of the mucous that lines and seals the intestinal wall. And that is good, because bacteria can enter the bloodstream through a leaking intestinal wall. Chevalier has therefore put her mice on a diet. Some of them receive a lot of dietary fiber, others very little. “This has produced exciting results. We see that the course of the disease is significantly milder in animals that receive high doses of fiber.” Chevalier and her team also succeeded in demonstrating that the immune system of mice fed a fiber-rich diet was activated much less frequently than that of the control group. Furthermore, the “fiber mice” were significantly slimmer. On the other hand, white fat synthesizes many inflammatory messengers, which in turn activate immune cells.
The mechanisms are highly complex. Immune cells, messengers, inflammatory mediators – everything is interconnected. Chevalier’s research explores the interplay between these factors and points the way to promising therapeutic approaches, whether with the help of drugs that can be used to block the pathogenic processes more specifically or through an adjustment of eating habits. The latter approach has the advantage of allowing patients to influence the success of the therapy – by eating less burgers.
Stephanie Streif

