Braking and Accelerating
Freiburg, Dec 18, 2018
Phosphatases are a type of enzyme that until recently were the orphans of science. Within the Cluster of Excellence CIBSS – Centre for Integrative Signaling Studies Prof. Dr. Maja Köhn aims to find out exactly how phosphatase act in signalling pathways, such as those that are triggered when the immune system encounters a cancer cell. Köhn, a phosphatase expert, builds biochemical tools. She hopes to discover new therapeutic approaches and to bring phosphatases out of the shadows.
 In order to make biochemical tools, Maja Köhn relies on her dual training – she is both a chemist and a biologist. Photo: Jürgen Gocke
In order to make biochemical tools, Maja Köhn relies on her dual training – she is both a chemist and a biologist. Photo: Jürgen Gocke
“We are toolmakers,” explains Maja Köhn. The tools she will produce in the CIBSS Cluster of Excellence are tiny molecules. And if used properly, they can inhibit or stimulate enzymes and other proteins – braking or accelerating their activity. Several CIBSS researchers will work with Köhn’s tools. She herself is interested in on a certain group of enzymes, the phosphatases. “They are fascinating,” says the molecular biologist, who was recruited to in 2016 by the BIOSS Excellence Cluster. Her group at the BIOSS Signalhaus investigates the role of phosphatases in signalling pathways - including those involved when the immune system responds to intruders or cancer cells.
Out of the shadows of science, into the spotlight
Until recently, phosphatases were the orphans of science. “Research had largely left them by the wayside,” says Köhn. It seemed of little point in investigating phosphatases – in contrast to. their adversaries, the kinases. Kinases add phosphate tags to other proteins. Phosphatases remove phosphates. The balance between the two counterparts – phosphatases and kinases – is a critical controlling element in many signal pathways. For example, it ensures that tissue and organs grow correctly in the human body, that injuries heal, and that the immune system attacks harmful bacteria and viruses. That means an imbalance between kinases and phosphatases can have serious consequences, such as developmental disorders, immunodeficiency or cancer.
|
Biological signals and signalling pathways Precise coordination is needed in order for trillions of cells to form, maintain and regenerate orderly tissues, organs and healthy organisms. To achieve this, complex communications processes take place between and within cells. Defects in these communications networks can lead to developmental disorders, immunodeficiency, cancer and other diseases. The CIBSS Cluster aims to achieve a comprehensive understanding of these communications processes – from the molecular level to the level of cells and organs. Furthermore, CIBSS research groups will investigate how these communications processes are connected to other important biological processes such as metabolism. Based on these new findings, the researchers will develop new possibilities to address challenges in areas as diverse as immunotherapy and the sustainable production of crops.
|
|
“Kinases were the stars of many scientific breakthroughs in the 1990s,” Köhn explains. Researchers discovered substances which specifically inhibited only a few kinases among the many in the body. That led to new medications for a number of diseases. Today, medicine uses these inhibitors to fight lung cancer, breast cancer, melanomas and tumors in the digestive tract, as well as autoimmune diseases such as rheumatoid arthritis. Kinase inhibitors have fewer side effects than the conventional medications because they act very specifically. “There are more than 30 inhibitors for kinases but almost none for phosphatases,” says Köhn. The reason: because the phosphatase inhibitors tested by scientists in the 1990s were not specific enough. They caused many side effects. Most researchers therefore lost interest in phosphatases.
Cancer suppresses immune cells to evade destruction
“Today we have new methods and new approaches for research,” Köhn says. “Especially with phosphatases, this gives us a lot of potential to exploit.” She mentions an example on which she has worked – the phosphatase PRL-3: “In healthy epithelium, such as in the gut, you simply don’t find any PRL-3.” But this is not the case if the tissue is degenerated, as is the case in cancer. “The more advanced the cancer or the metastases are, the more PRL-3 we find. The phosphatase disrupts the order in the epithelium tissue.” A lack of order in a tissue means chaotic, uncontrolled growth – and usually cancer. Köhn and her group have elucidated PRL-3’s biochemistry and have developed an inhibitor for the phosphatase. “Under its influence, order in the tissue is restored,” she says.
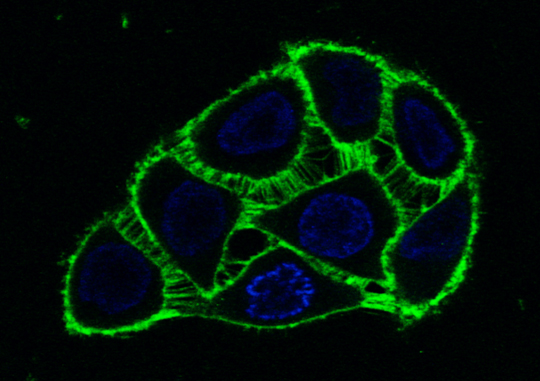 The peptide (green) that activates the phosphatase can be seen at the membrane in living cells. Source: Jeremy Chojnacki
The peptide (green) that activates the phosphatase can be seen at the membrane in living cells. Source: Jeremy Chojnacki
Now Köhn intends to explore new terrain. “We want to understand the role of phosphatases in immune receptor signalling pathways,” she says. She gives another example: Tumor cells can express a specific molecule on their surface – in other words, they can present a bound signalling protein. This molecule binds to T cells, a kind of immune cell. When this happens, a signalling pathway is engaged in the T cells that prevents them from attacking the tumor. “Phosphatases play a big role in this process. We want to explore these signalling pathways in detail and find out what exactly the phosphatases are doing.” In order to reach this goal, she is building molecular tools. With the right inhibitors Köhn would be able to control the signal pathways very precisely in space and time. She could influence in which part of the signalling pathway one or more given phosphatases are active, and control the degree of their activity.
Perfect bonds should lead to new therapies
“We know the structure of the enzymes fairly precisely and we know what they look like three-dimensionally,” Köhn explains. “That tells us a lot about their properties.” Using a computer model, she can see how a phosphatase bonds its natural partner, the target protein from which it chops off a phosphate. “Sometimes there are gaps in the bonding – weak points,” says Köhn. Then her team members can use synthetic chemistry to make molecules which fit better and bind more tightly. Experiments in the test tube and in cell culture then show whether the molecule bonds strongly enough. Can it block the docking site on the phosphatase, thereby inhibiting the enzyme?
 Golden and crystal clear: The researchers produce peptides from specially prepared building blocks. Photo: Jürgen Gocke
Golden and crystal clear: The researchers produce peptides from specially prepared building blocks. Photo: Jürgen Gocke
Alternatively, the working group can carry out mutation studies. They create many variations of a phosphatase bonding partner and test each one to see how strongly it binds. “Then we cut out the docking site from the best bonding partner and build it into a new molecule,” says Köhn. If that works as planned, the docking point can be blocked and the corresponding phosphatase will be inhibited. “In the long run I hope that one of our molecules will spur the development of new bioactive molecules.” After all, why shouldn’t it be possible to do with phosphatases what has long been done with kinases? Some of the molecules are set to be tested in preclinical animal models within the Cluster of Excellence. “I hope they will work against cancer,” says Köhn. That would be the first step on the road to new treatments.
Two scientific disciplines, one career
Reaching this milestone will mean a lot of hard work for Köhn’s twelve-person working group. “Half are working in molecular and cell biology, the other half more in synthetic chemistry,” says the group leader. Her career also unites two disciplines. She started out studying chemistry in Kiel and completed her doctorate in Organic Chemistry. Via Harvard, Köhn arrived at the European Molecular Biology Laboratory EMBL in Heidelberg: “That’s where I became a biologist.” Since 2016 she has been conducting research in Freiburg. And Maja Köhn has set a clear goal for her career: “I would hope phosphatases will be established as target proteins for new therapeutic agents.”
Jürgen Schickinger
CIBSS – Centre for Integrative Biological Signaling Studies
CIBSS is one of the two new Clusters of Excellence at the University of Freiburg. In CIBSS, more than 60 working groups will investigate biological signalling processes of immune cells and mitochondria, and in organ development and in plant roots. The researchers come from six faculties – for Biology, Mathematics and Physics, Medicine, Chemistry and Pharmacy, Engineering, and Law. The University Medical Center and the Max Planck Institute of Immunobiology and Epigenetics are also participating in CIBSS. The German Research Foundation (DFG) is funding the Cluster from the beginning of 2019 until the end of 2025.
CIBSS – Centre for Integrative Biological Signaling Studies